Eustrombus gigas
| Eustrombus gigas | |
|---|---|
 |
|
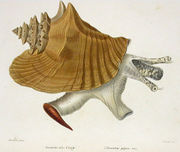
| A subadult individual of Eustrombus gigas, in situ surrounded by turtle grass | |
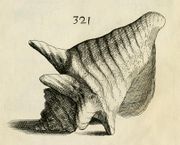
 |
|
| A dorsal view of an adult individual of E. gigas from Kiener, 1834 | |
| Conservation status | |
|
Not evaluated (IUCN 3.1)[1]
|
|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Gastropoda |
| (unranked): | clade Caenogastropoda clade Hypsogastropoda clade Littorinimorpha |
| Superfamily: | Stromboidea |
| Family: | Strombidae |
| Genus: | Eustrombus |
| Species: | E. gigas |
| Binomial name | |
| Eustrombus gigas (Linnaeus, 1758) |
|
| Synonyms[9] | |
|
Strombus gigas Linnaeus, 1758[2] |
|
Eustrombus gigas, commonly known as the queen conch, is a species of very large edible sea snail, a marine gastropod mollusk in the family of true conchs, the Strombidae. This is one of the largest mollusks native to the Tropical Northwestern Atlantic, from Bermuda to Brazil. Other common names for the species include the pink conch, caracol reina, caracol rosa, caracol rosado, caracol de pala, cobo, botuto, guarura and lambí.[10][11][12][13] This large herbivorous gastropod lives in seagrass beds, although the exact habitat varies during the different stages of its development. The adult animal has a very large, solid and heavy shell, with knob-like spines on the shoulder, a flared thick outer lip and a characteristic pink-colored aperture (opening). The flared lip is completely absent in younger specimens. The external anatomy of the soft parts of E. gigas is similar to that of other Strombidae snails; it has a long snout, two eyestalks with well-developed eyes and additional sensory tentacles, a strong foot and a corneous sickle-shaped operculum.
Eustrombus gigas is home to several commensal animals including slipper snails, porcelain crabs and cardinal fish. Its parasites include coccidians. The queen conch is hunted and eaten by several other species of large sea snail, and also by starfish, crustaceans and vertebrates (fish, sea turtles and humans). The meat of this sea snail is consumed by humans in a wide variety of dishes. The shell is sold as a souvenir and used as a decorative object. Historically, Native Americans and indigenous Caribbean peoples used parts of the shell of this species to form various tools.
The queen conch is protected under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) agreement,[14] in which it is listed as Strombus gigas. This species is not yet truly endangered in the Caribbean as a whole, but it is commercially threatened in numerous areas. This is partly because of extreme overfishing; the meat is an important food source for humans. The CITES regulations are designed to halt the export of the meat of this species as well as the commercial export of the shells as decorative objects. Both of these trades were previously so prevalent that they represented serious threats to the survival of the species.
Contents |
Taxonomy
This sea snail was originally described in 1758 by the Swedish naturalist and taxonomist Carl Linnaeus. The specific name was taken from the Ancient Greek word gigas (γίγας), which means "giant", alluding to its large size compared with almost all other gastropod mollusks. Linnaeus named the species Strombus gigas and that remained its name for over two hundred years. However, the family Strombidae has recently undergone an extensive taxonomic revision, and a few subgenera, including Eustrombus, were elevated to genus level by some authors.[15][16][17] Petuch (2004)[18] and Petuch and Roberts (2007)[19] recombined this species as Eustrombus gigas, and Landau et al. (2008) recombined it as Lobatus gigas.[17]
Linnaeus did not mention a specific locality in his original description, giving only "America" as the area in which the species is found. Over the centuries no type material (the shell or shells on which he based his description) survived, and thus there was no type material for scientists to refer to. In 1941, the type of this species was officially designated by the American malacologists Clench and Abbott. In this case, the type is not an actual specimen, but a figure from the book Recreatio mentis, et occuli by the Italian scholar Filippo Buonanni, published in 1684. Also designated by Clench and Abbott, the type locality of E. gigas is Montego Bay, Jamaica.[20][21][22]
Shell description
.jpg)
The adult shell of this species is usually 15–31 cm (6–12 inches) in length[23][24] (maximum reported size reaches 35.2 cm).[9][20] It is very solid and heavy, with 9 to 11 whorls and a widely flaring and thickened outer lip.[20] In the shells of adult snails, a structure called the stromboid notch is present on the edge of the lip. Although this notch is not as well developed in this species as it is in other species in the same family,[20] the shell feature is nonetheless visible in an adult dextral (normal right-handed) specimen, as a secondary anterior indentation in the lip of the shell, to the right of the siphonal canal, assuming the shell is viewed ventrally. In life, the animal's left eyestalk protrudes through this notch.[20][25][26][27]
The spire (a protruding part of the shell which includes all of the whorls with exception of the largest final whorl, known as the body whorl) is usually higher (more elongated) in this species than it is in the shells of other strombid snails, such as the closely related and even larger goliath conch, Eustrombus goliath, a species endemic to Brazil.[20] The glossy finish around the aperture of the adult shell is colored primarily in shades of pink. This pink glaze is usually pale, and may show a cream, peach or yellow coloration, but it can also be tinged with a deep magenta, shading almost to red. The periostracum, an organic coating covering the shell's surface, is thin and a pale brown color in this species.[24][25][26]
The overall shell morphology of E. gigas is not solely determined by the animal's genes, because environmental conditions (such as geographic location, food supply, temperature and depth) and biological interactions (such as exposure to predation) can greatly affect it.[28][29] Juvenile conchs develop heavier shells when exposed to predators compared to those that are not exposed to predators. Conchs also develop wider and thicker shells with fewer but longer spines when they are living in deeper water.[29]
The shells of very small juvenile queen conchs are strikingly different in appearance from those of the adults. Noticeable is the complete absence of a flared outer lip; juvenile shells have a simple sharp lip, which gives the shell a conical or biconic outline. In Florida, juvenile queen conchs are known as "rollers", because wave action very easily rolls their shells, whereas it is nearly impossible to roll an adult specimen, due to its weight and asymmetrical profile. Subadult shells have a flared lip which is very thin. The flared outer lip of an adult shell gradually increases its thickness with age.[30][31][32]
Early illustrations from classic shell books
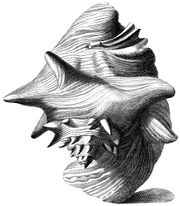
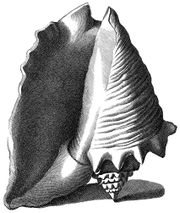
Index Testarum Conchyliorum (published in 1742 by the Italian physician and malacologistNiccolò Gualtieri) has illustrations showing the morphology of adult queen conch shells from different perspectives.
 |
 |
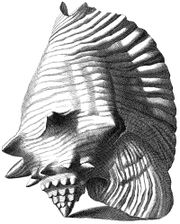 |
In each of the illustrations, the knobbed spire and the flaring outer lip, with its somewhat wing-like contour expanding out from the last whorl, is a striking feature. The shells are shown as if balancing on their apex and the edge of the lip.

A much later colored illustration from the Manual of Conchology (published in 1885 by the American malacologist George Washington Tryon) shows a dorsal view of a small juvenile shell with its typical brown and white patterning.[32]
Anatomy

Many details about the anatomy of Eustrombus gigas were not well known until 1965, when the zoologist Colin Little published a general study on the subject.[33] More recently, in 2005, the malacologist Luiz R. L. Simone gave a detailed anatomical description of the species.[16] Eustrombus gigas has a long extensible snout (which interiorly contains a taenioglossan type radula),[33] with two eyestalks (also known as ommatophores) that originate from its base. The tip of each eyestalk contains a large well developed lens eye, with a black pupil and a yellow iris[20] (if amputated, its eyes can be completely regenerated),[34] and a small slightly posterior sensory tentacle.[23]
Both the snout and the eyestalks show dark spotting in the exposed areas. The mantle is darkly colored at the anterior region, fading to light gray at the posterior end, while the mantle collar is commonly orange and the siphon is also orange or yellow.[33]
When the soft parts of the animal are removed from the shell, several organs are distinguishable externally, including the kidney, the nephiridial gland, the pericardium, the genital glands, stomach, style sac, and the digestive gland.[33]

Eustrombus gigas has a large and powerful foot, which has brown spots and markings towards the edge, but is white nearer to the visceral hump (the part of the animal that stays inside, protected by the shell, and which accommodates a number of internal organs). The base of the anterior end of the foot has a distinct groove, which contains the opening of the pedal gland. Attached to the posterior end of the foot for about one third of its length is the dark brown, corneous, sickle-shaped operculum, which is reinforced by a distinct central rib. The base of the posterior two-thirds of the animal's foot is rounded; only the anterior third of the foot is applied to the substrate during locomotion.[33][16]
The columella, the central pillar within the shell, serves as the attachment point for the white collumellar muscle. Contraction of this large strong muscle allows all of the soft parts of the animal to withdraw into the shell in response to undesirable stimuli.[33]
Distribution
Eustrombus gigas is native to the tropical Western Atlantic coasts of North and Central America.[26] It lives in the greater Caribbean tropical zone, at depths from 0.3 m to 18 m,[26] and the countries and regions where this species is recorded as having been found include (in alphabetical order):[9][35][36]

Aruba, of the Netherland Antilles; Barbados; Bimini, Cat Island, Eleuthera, Grand Bahama Island, Inagua (Great or Little) and Little San Salvador in the Bahamas; Belize; Bermuda; North and northeastern regions of Brazil (though this is contested by some authors);[20] Old Providence Island in Colombia; Costa Rica; the Dominican Republic; Panama; Swan Islands in Honduras; Jamaica; Martinique; Alacran Reef, Campeche, Cayos Arcas and Quintana Roo, in Mexico; Puerto Rico; Saint Barthélemy; Mustique and Grenada in the Grenadines; Pinar del Rio, North Havana Province, North Matanzas, Villa Clara, Cienfuegos, Holguin, Santiago de Cuba and Guantanamo, in the Turks and Caicos Islands and Cuba; South Carolina, Florida, including East Florida, West Florida, the Florida Keys and the Flower Garden Banks National Marine Sanctuary, in the USA; Carabobo, Falcon, Gulf of Venezuela, Los Roques archipelago, Los Testigos Islands and Sucre in Venezuela; St. Croix in the Virgin Islands.
Behavior
Eustrombus gigas has an unusual means of locomotion, which was described in 1922 by the American zoologist George Howard Parker (1864–1955).[37][38] The animal first fixes the posterior end of the foot by thrusting the point of the sickle-shaped operculum into the substrate, then it extends the foot in a forward direction, lifting and throwing the shell forward in a so-called leaping motion. This way of moving is considered to resemble that of pole vaulting,[39] making E. gigas a good climber even of vertical concrete surfaces,[40] and may also help prevent predators from following its chemical traces over the substrate.[41]
Ecology

Habitat
Eustrombus gigas lives in seagrass meadows and on sandy substrate,[42] usually in association with turtle grass (species of the genus Thalassia, namely Thalassia testudinum[30] and also Syringodium sp.)[28] and manatee grass (Cymodocea sp.).[43] Juvenile individuals are found in shallow, inshore seagrass meadows, which are different from the deeper algal plains and seagrass meadows where adult individuals live.[26][44]
The critical nursery habitats for juvenile individuals are defined by a series of combined factors, both habitat characteristics (such as tidal circulation) and ecological processes (such as macroalgal production), which together provide high rates of both recruitment and survival.[45] Eustrombus gigas is typically found in distinct aggregates, which may contain several thousand individuals.[29]
Life cycle
Eustrombus gigas is dioecious, which means each individual snail is either distinctly male or distinctly female.[27] Females are usually larger than males in natural populations, with both sexes existing in similar proportion.[42] After internal fertilization,[29] the females lay eggs in gelatinous strings, which can be as long as 75 feet.[26] These are layered on patches of bare sand or seagrass. The sticky surface of these long egg strings allows them to coil and agglutinate, mixing with the surrounding sand to form compact egg masses, which shape is defined by the anterior portion of outer lip of the female's shell while they are layered.[29][46] Each one of the egg masses may have been fertilized by multiple males.[46] The number of eggs per egg mass may vary greatly depending on environmental conditions, such as food limitation and temperature.[29][46] Commonly, females produce an average of 8–9 egg masses per season,[27][47] each containing 180,000–460,000 eggs,[26] but numbers can be as high as 750,000 eggs per egg mass depending upon the conditions.[29] Eustrombus gigas females may spawn multiple times during the reproductive season,[26] which lasts from March to October, with activity peaks occurring from July to September.[27]
After hatching, the emerging two-lobed veliger (a larval form common to various marine and fresh-water gastropod and bivalve mollusks)[48] spend several days developing in the plankton, feeding primarily on phytoplankton. Metamorphosis occurs in about 16–40 days from the hatching,[29] when the fully grown protoconch (embryonic shell) is about 1.2 mm high.[42] After the metamorphosis, Eustrombus gigas individuals spend the rest of their lives in the benthic zone (on or in the sediment surface), usually remaining buried during their first year of life.[49]
The queen conch is known to reach sexual maturity at approximately 3 to 4 years of age, reaching a shell length of nearly 180 mm and weighting up to 5 pounds.[26][27] Individuals may usually live up to 7 years, though in deeper waters their lifespan may reach 20–30 years[26][29][42] and maximum lifetime estimates reach 40 years.[50] It is believed that mortality rate tends to be lower in matured conchs due to their thickened shell, but it could be substantially higher for juveniles. Estimates have demonstrated that the mortality rate of E. gigas decreases as the animal size increases, and can also vary due to habitat, season and other factors.[49]

Feeding habits
Strombid gastropods were widely accepted as carnivores by several authors in the 19th century, a conception that persisted until the first half of the 20th century. This erroneous idea probably originated in the writings of Lamarck, who classified strombids alongside other supposedly carnivorous snails, and was posteriorly recovered by other authors. The claims of such authors, however, were never supported by in situ observations.[51] Eustrombus gigas is now known to be a specialized herbivore,[24] as is the case in other Strombidae,[51][15] feeding on macroalgae (including red algae, such as species of Gracilaria and Hypnea),[32] seagrass[43] and unicellular algae, intermittently also feeding on algal detritus.[51][52] The green macroalga Batophora oerstedii is one of its preferred foods.[26]
Interspecific relationships

A few different animals may establish a commensal interaction with E. gigas, which means both the organisms maintain a relationship where one individual benefits (the commensal) but the other obtains no advantage (in this case, the queen conch). Some commensals of this species are also mollusks, mainly slipper shells (Crepidula spp.).[28] The porcelain crab, Porcellana sayana, is also known to be a commensal,[28] and a small cardinal fish, known as the conch fish (Astrapogon stellatus),[28] sometimes lives in the mantle of the conch for protection, bringing E. gigas no apparent benefit.[26] E. gigas is very often parasitized by protists of the phylum Apicomplexa,[53][54] which are common parasites of mollusks. Those coccidian[53] parasites, which are spore-forming, single-celled microorganisms, initially establish themselves in large vacuolated cells of the hosts digestive gland, where they reproduce freely.[53][54] The infestation may proceed to the secretory cells of the same organ, and the entire life cycle of the parasite will likely occur within the same host and tissue.[53]

E. gigas is a prey species for several carnivorous gastropod mollusks, including the apple murex Chicoreus pomum, the horse conch Pleuroploca gigantea, the lamp shell Turbinella angulata, the moon snails Natica spp. and Polinices spp., the muricid snail Murex margaritensis, the trumpet triton Charonia variegata and the tulip snail Fasciolaria tulipa.[20][23][55] A variety of crustaceans are also known predators of conchs, such as the blue crab Callinectes sapidus, the box crab Calappa gallus, the giant hermit crab Petrochirus diogenes, the spiny lobster Panulirus argus and several other species.[23][55] The queen conch is also prey to echinoderms, such as the cushion star, Oreaster reticulatus, and vertebrates, including fish (such as the permit Trachinotus falcatus[56] and the porcupine fish Diodon hystrix), loggerhead sea turtles (Caretta caretta) and humans.[23][55]
Human uses

Conch meat has been eaten by humans for centuries, and has traditionally been an important part of the diet in many islands in the West Indies. It is consumed raw, marinated, minced or chopped in a wide variety of dishes, such as salads, chowder, fritters, soups, stew, pâtés and other local recipes.[23][39][43][57] In the Spanish-speaking regions, for example in the Dominican Republic, Eustrombus gigas meat is known as lambí. Although the queen conch meat is used mainly for human consumption, it is also sometimes employed as fishing bait (usually the foot is utilized for such purpose).[43][50] E. gigas is among the most important fishery resources in the Caribbean: its harvest value was US$30 million in 1992,[29] increasing to $60 million in 2003.[58] The total annual harvest of meat of E. gigas ranged from 6,519,711 kg to 7,369,314 kg between 1993 and 1998, and later its production declined to 3,131,599 kg in 2001.[58] Data about imports of queen conch meat into the United States shows a total of 1,832,000 kg in 1998, as compared to 387,000 kg in 2009, a drastic reduction of nearly 80%, twelve years later.[59]

Queen conch shells were used by Native Americans and Caribbean Indians in a wide variety of ways. The South Florida Indians (such as the Tequesta), the Carib, the Arawak and Taino used conch shells to fabricate tools (such as knives, axe heads and chisels), jewelry, cookware and also used them as blowing horns.[23][60] Aztecs used the shell as part of jewelry mosaics like the double-headed serpent.[61] Brought by explorers, queen conch shells quickly became a popular asset in early modern Europe. In the late 17th century they were widely used as decoration over fireplace mantels and English gardens, among other places.[39] In contemporary times, queen conch shells are mainly utilized in handicraft. Shells are made into cameos, bracelets and lamps,[43][62] but have also been traditionally used as doorstops or decorations by families of seafaring men.[62] The shell of the queen conch has been, and continues to be, popular as a decorative object, though its export is now regulated and restricted by the CITES agreement.[23]
Very rarely (about 1 in 10,000 conchs),[23] a pink-colored conch pearl[31] may be found within the mantle of the animal.[23] Those pearls are considered semi-precious,[20] a popular tourist curio,[43] and the most attractive among them have been used to create necklaces and earrings. A conch pearl is a non-nacreous pearl (formerly referred to by some sources as a 'calcareous concretion' – see the pearl article), which differs from most pearls sold as gemstones.[63]
Threats and conservation measures
Within the conch fisheries, one of the threats to sustainability stems from the fact that there is almost as much meat in large juveniles as there is in adults, but only adult conchs can reproduce and thus build up a population.[57] In many places where adult conchs have become rare due to overfishing, larger juveniles and subadult animals are taken by the fishermen before the snails have had a chance to mate and lay eggs.[57][64]
On a number of islands, subadult conchs form the vast majority of the harvest.[65] The abundance of Eustrombus gigas is declining throughout the species' range as a result of overfishing and poaching, and populations of the species in Honduras, Haiti and the Dominican Republic, in particular, are currently being exploited at rates that may be unsustainable.[50] In fact, trade from many Caribbean countries is known or suspected to be unsustainable, and illegal harvest, including fishing of the species in foreign waters and subsequent illegal international trade, is a common and widespread problem in the region.[50] The Caribbean "International Queen Conch Initiative" is an attempt at a fisheries management scheme for this species.[36]
The queen conch fishery is usually managed under the regulations of individual nations. In the United States all taking of queen conch is prohibited in Florida and in adjacent Federal waters. No international regional fishery management organization exists in the whole Caribbean area, but in places such as Puerto Rico and the Virgin Islands, queen conch is regulated under the auspices of the Caribbean Fishery Management Council (CFMC).[50] In 1990, the Parties to the Convention for the Protection and Development of the Marine Environment of the Wider Caribbean Region (Cartagena Convention) included queen conch in Annex II of its Protocol Concerning Specially Protected Areas and Wildlife (SPAW Protocol) as a species that may be used on a rational and sustainable basis and that requires protective measures. Because of this recognition, in 1992 the United States proposed queen conch for listing in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES); this proposal was adopted, and queen conch became the first large-scale fisheries product to be regulated by CITES.[50] Although this species has been mentioned in CITES since 1985[29] and has been listed in Appendix II[14] since 1992,[66] it is only since 1995 that CITES has been reviewing the biological and trade status of the queen conch under its "Significant Trade Review" process. Significant Trade Reviews are undertaken when there is concern about levels of trade in an Appendix II species. Based on the 2003 review,[58] CITES recommended that all countries prohibit the importation of queen conch from Honduras, Haiti and the Dominican Republic, according to Standing Committee Recommendations.[67] Queen conch meat continues to be available from many other Caribbean countries, including Jamaica and the Turks and Caicos Islands (British West Indies), which have well-managed queen conch fisheries.[50]
References
This article incorporates public domain text (a public domain work of the United States Government) from the reference.[50]
- ↑ IUCN (2009). IUCN Red List of Threatened Species <www.iucnredlist.org>. Retrieved 13 April 2010.
- ↑ Linnaeus, C. (1758). Systema Naturae, 10th ed., vol. 1. 824 pp. Laurentii Salvii: Holmiae (Stockholm, Sweden). p. 745.
- ↑ 3.0 3.1 Linnaeus, C. (1758). Systema Naturae, 10th ed., vol. 1. 824 pp. Laurentii Salvii: Holmiae (Stockholm, Sweden). p 744.
- ↑ Clench, W. J. (1937). "Descriptions of new land and marine shells from the Bahama Islands." Proceedings of the New England Zoölogical Club 16: 17–26, pl. 1. (Stated date: 5 February 1937.) On pages 18–21, plate 1 figure 1.
- ↑ Smith, M. (1940). World Wide Seashells: illustrations, geographical range and other data covering more than sixteen hundred species and sub-species of molluscs (1 ed.). Lantana, Florida: Tropical Photographic Laboratory. p. 131.
- ↑ McGinty, T. L. (1946). "A new Florida Strombus, S. gigas verrilli". The Nautilus 60: 46–48, plates. 5–6: plate 5, figs. 2–3; plate 6, figs. 7–8.
- ↑ Burry, L. A. (1949). Shell Notes (Lantana, Florida: Frank Lyman) 2: 106-109.
- ↑ Petuch, E. J. (1994). Atlas of Florida Fossil Shells. Chicago Spectrum Press: Evanston, Illinois., xii + 394 pp., 100 pls. On page 82, plate 20: figure c.
- ↑ 9.0 9.1 9.2 Eustrombus gigas (Linnaeus, 1758). A Database of Western Atlantic Marine Mollusca, Malacolog Version 4.1.1.. Retrieved 27 September 2009.
- ↑ Buitriago, J. (1983). "Cria en cautiverio, del huevo al adulto, del botuto (Strombus gigas L)". Memoria Sociedad de Ciencias Naturales La Salle 43: 29–39. http://orton.catie.ac.cr/cgi-bin/wxis.exe/?IsisScript=AGRINVE.xis&method=post&formato=2&cantidad=1&expresion=mfn=003428.
- ↑ Avalos, D. C. (1988). "Crecimiento y mortalidad de juveniles de Caracol rosado Strombus gigas en Punta Gavilán, Q. Roo". Documentos de Trabajo (Mexico: Secretaria de Pesca) 16: 1–16. http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=REPIDISCA&lang=p&nextAction=lnk&exprSearch=105074&indexSearch=ID.
- ↑ Posada, J. M.; Ivan, M. R. & Nemeth, M. (1999). "Occurrence, abundance, and length frequency distribution of queen conch, Strombus Gigas, (Gastropoda) in shallow waters of the Jaragua National Park, Dominican Republic". Caribbean Journal of Science 35 (1–2): 70–82. http://www.cavehill.uwi.edu/FPAS/bcs/courses/Ecology/BL34B/fish5-3.pdf.
- ↑ Eustrombus gigas (Linnaeus, 1758). World Register of Marine Species, accessed 13 August 2010.
- ↑ 14.0 14.1 Appendices I, II and III. cites.org website. Retrieved 4 July 2009.
- ↑ 15.0 15.1 Latiolais, J. M.; Taylor, M. S; Roy, K. & Hellberg, M. E. (2006). "A molecular phylogenetic analysis of strombid gastropod morphological diversity". Molecular Phylogenetics and Evolution (Elsevier) 41 (2): 436–444. doi:10.1016/j.ympev.2006.05.027. PMID 16839783. http://cstl-csm.semo.edu/mtaylor/pubs/Latiolaisetal2006.pdf.
- ↑ 16.0 16.1 16.2 Simone, L. R. L. (2005). "Comparative morphological study of representatives of the three families of Stromboidea and the Xenophoroidea (Mollusca, Caenogastropoda), with an assessment of their phylogeny". Arquivos de Zoologia (São Paulo: Museu de Zoologia – USP) 37 (2): 178–180. ISSN 0066-7870. http://www.revistasusp.sibi.usp.br/pdf/azmz/v37n2/a01v37n2.pdf.
- ↑ 17.0 17.1 Landau, B. M.; Kronenberg G. C.; Herbert, G. S. (2008). "A large new species of Lobatus (Gastropoda: Strombidae) from the neogene of the Dominican Republic, with notes on the genus". The Veliger (Santa Barbara: California Malacozoological Society, Inc.) 50 (1): 31–38. ISSN 0042-3211.
- ↑ Petuch, E. J. (2004). Cenozoic Seas: The view from Eastern North America. CRC Press. pp. 1–308. ISBN 0-8493-1632-4. http://books.google.com/?id=K0hVJTLfGbMC&printsec=frontcover&dq=Cenozoic+Seas:+The+View+from+Eastern+North+America&q.
- ↑ Petuch, E. J.; Roberts, C. E. (2007). The geology of the Everglades and adjacent areas. Boca Raton, Florida: CRC Press, Taylor & Francis Group. pp. 1–212. ISBN 1-4200-4558-X. http://books.google.com/?id=_RlF61pBkqQC&printsec=frontcover&dq=petuch+geology+of+everglades&q.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 Moscatelli, R. (1987). The superfamily Strombacea from Western Atlantic. São Paulo, Brazil: Antonio A. Nanô & Filho Ltda. pp. 53–60.
- ↑ Buonanni, F. (1684). Recreatio mentis, et oculi in observatione animalium testaceorum Italico sermone primum proposita ... nunc ... Latine oblata centum additis testaceorum iconibus. Romae, Ex typographia Varesii. pp. 531–532. http://www.archive.org/stream/recreatiomentise00buon#page/n531/mode/2up.
- ↑ Clench, W. J.; Abbott, R. T. (1941). "The genus Strombus in the Western Atlantic". Johnsonia 1 (1): 1–16.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 23.9 Toller, W.; Lewis, K-A. (2003). Queen Conch Strombus gigas. U.S.V.I. Animal Fact Sheet. 19. U.S.V.I. Department of Planning and Natural Resources Division of Fish and Wildife. http://fw.dpnr.gov.vi/education/FactSheets/PDF_Docs/19QueenConch.pdf.
- ↑ 24.0 24.1 24.2 Warmke, G. L.; Abbott, R. T. (1975). Caribbean Seashells. Dover Publications, Inc. p. 88. ISBN 0-486-21359-5.
- ↑ 25.0 25.1 Leal, J. H. (2002). "Gastropods". In Carpenter, K. E.. The living marine resources of the Western Central Atlantic. Volume 1: Introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaeras. FAO Species Identification Guide for Fishery Purposes. FAO. p. 139. ftp://ftp.fao.org/docrep/fao/009/y4160e/y4160e09.pdf.
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 26.10 26.11 "Smithsonian Marine Station at Fort Pierce". Strombus gigas. Smithsonian Institution. http://www.sms.si.edu/IRLSpec/Strombus_gigas.htm. Retrieved 2009-10-26.
- ↑ 27.0 27.1 27.2 27.3 27.4 Davis, M. (2005). "Species Profile: Queen Conch, Strombus gigas". SRAC Publication No. 7203 (Southern Region Aquaculture Center). http://www.ca.uky.edu/wkrec/QueenConch.pdf.
- ↑ 28.0 28.1 28.2 28.3 28.4 Tewfik, A. (1991)."An assessment of the biological characteristics, abundance, and potential yield of the queen conch (Strombus gigas L.) fishery on the Pedro Bank off Jamaica". Thesis submitted in partial fulfillment of the requirements for the Degree of Masters of Science (Biology). Acadia University, Canada.
- ↑ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09 29.10 McCarthy, K. (2007). "A review of queen conch (Strombus gigas) life-history. Sustainable Fisheries Division NOAA. SEDAR 14-DW-4.
- ↑ 30.0 30.1 Davis, J.E. (2003). "Population assessment of queen conch, Strombus gigas, in the St. Eustatius Marine Park, Netherlands Antilles". St. Eustatius Marine Park.
- ↑ 31.0 31.1 Abbott, R. T. (2002). Zim, H. S.. ed. Seashells of The World. New York: St. Martin's Press. ISBN 1-58238-148-8.
- ↑ 32.0 32.1 32.2 Tryon, G. W. (1885). Manual of Conchology, structural and systematic, with illustrations of the species. Volume 7. Terebridae, Cancellariidae, Strombidae, Cypraeidae, Ovulidae, Cassididae, Doliidae. pp. 107; 348.
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 Little, C. (1965). "Notes on the anatomy of the queen conch, Strombus gigas". Bulletin of Marine Science 15 (2): 338–358. abstract.
- ↑ Hughes, H. P. I. (1976). "Structure and regeneration of the eyes of strombid gastropods". Cell and Tissue Research 171 (2): 259–271. PMID 975213.
- ↑ Martin-Mora, E.; James, F. C.; Stoner, A. W. (1995). "Developmental plasticity in the shell of the queen conch Strombus gigas". Ecology (Ecological Society of America) 76 (3): 981–994. doi:10.2307/1939361. http://www.jstor.org/pss/1939361.
- ↑ 36.0 36.1 "International Queen Conch Initiative". NOAA: Caribbean Fishery Management Council. http://www.strombusgigas.com. Retrieved 2009-09-27.
- ↑ Coan, E. V.; Kabat, A. R.; Petit, R. E. (2010). 2,400 Years of Malacology (7th ed.). American Malacological Society. p. 874. http://www.malacological.org/pdfs/2400years/2400yrs_of_Malacology_complete.pdf.
- ↑ Parker, G. H. (1922). "The leaping of the stromb (Strombus gigas Linn.)". Journal of Experimental Zoology 36 (2): 205–209. doi:10.1002/jez.1400360204. http://www3.interscience.wiley.com/journal/110509814/abstract?CRETRY=1&SRETRY=0.
- ↑ 39.0 39.1 39.2 "Queen conch: Florida’s spectacular sea snail". Sea Stats. Florida Fish and Wildlife Conservation Commission. 2006. http://research.myfwc.com/engine/download_redirection_process.asp?file=queenconch_4434.pdf&objid=-1595&dltype=product. Retrieved 31 August 2010.
- ↑ Hesse, K. O. (1980). "Gliding and climbing behaviour of the queen conch, Strombus gigas". Caribbean Journal of Science 16: 105–108. http://academic.uprm.edu/publications/cjs/VOL16/P105-107.PDF.
- ↑ Berg, C. J. (1975). "Behavior and ecology of conch (superfamily Strombacea) on a deep subtidal algal plain". Bulletin of Marine Science 25 (3): 307–317. abstract.
- ↑ 42.0 42.1 42.2 42.3 Ulrich Wieneke (ed.). Lobatus gigas. In: Gastropoda Stromboidea. modified: 22 November 2008. Retrieved 23 June 2009.
- ↑ 43.0 43.1 43.2 43.3 43.4 43.5 "Gastropods". FAO Identification Sheets for Fishery Purposes: Western Central Atlantic (fishing area 31). Food and Agriculture Organization of the United Nations (FAO). http://www.strombusgigas.com/strombus%20species%20id%20pg2.pdf.
- ↑ Stoner, A. W. (1988). "Winter mass migration of juvenile queen conch Strombus gigas and their influence on the benthic environment". Marine Ecology Progress Series 56: 99–104.
- ↑ Stoner, A. W. (2003). "What constitutes essential nursery habitat for a marine species? A case study of habitat form and function for queen conch". Marine Ecology Progress Series (Inter-Research) 257: 275–289. doi:10.3354/meps257275. ISSN 1616-1599. http://cat.inist.fr/?aModele=afficheN&cpsidt=15047378.
- ↑ 46.0 46.1 46.2 Robertson, R. (1959). "Observations on the spawn and veligers of conchs (Strombus) in the Bahamas". Proceedings of the Malacological Society of London 33 (4): 164–171.
- ↑ Davis, M.; Hesse, C.; Hodgkins, G. (1987). "Commercial hatchery produced queen conch, Strombus gigas, seed for the research and grow-out market". Proceedings of the Gulf and Caribbean Fisheries Institute (Gulf and Caribbean Fisheries Institute) 38: 326–335.
- ↑ Brusca, R. C.; Brusca, G. J. (2003). Invertebrates (2nd ed.). Sinauer Associates, Inc.. p. 936. ISBN 0878930973.
- ↑ 49.0 49.1 Medley, P. (2008). Monitoring and managing queen conch fisheries: A manual. FAO Fisheries Technical Paper. 514. Rome: Food and Agriculture Organization of the Uniteded Nations (FAO). ISBN 978-92-5-106031-5. ftp://ftp.fao.org/docrep/fao/011/i0256e/i0256e.pdf.
- ↑ 50.0 50.1 50.2 50.3 50.4 50.5 50.6 50.7 NOAA. Queen Conch (Strombus gigas). Retrieved 4 July 2009.
- ↑ 51.0 51.1 51.2 Robertson, R. (1961). "The feeding of Strombus and related herbivorous marine gastropods". Notulae Naturae of the Academy of Natural Sciences of Philadelphia (343): 1–9.
- ↑ Stoner, A.; Ray, M. (1996). "Queen conch, Strombus gigas, in fished and unfished locations of the Bahamas: effects of a marine fishery reserve on adults, juveniles, and larval production". Fishery Bulletin (USA: National Oceanic and Atmospheric Administration (NOAA)) 94 (3): 551–565. http://fishbull.noaa.gov/943/stoner.pdf.
- ↑ 53.0 53.1 53.2 53.3 Cárdenas, E. B.; Frenkiel, L.; Zarate, A. Z.; Aranda, D. A. (2007). "Coccidian (Apicomplexa) parasite infecting Strombus gigas Linné, 1758 digestive gland". Journal of Shellfish Research (NSA) 26 (2): 319–321. doi:10.2983/0730-8000(2007)26[319:CAPISG]2.0.CO;2. http://www.thefreelibrary.com/Coccidian+(Apicomplexa)+parasite+infecting+Strombus+gigas+Linne,+1758+...-a0168820706.
- ↑ 54.0 54.1 Gros, O.; Frenkiel, L.; Aranda, D. A. (2009). (Abstract) "Structural analysis of the digestive gland of the queen conch Strombus Gigas Linnaeus, 1758 and its intracellular parasites". Journal of Molluscan Studies (UK: Oxford University Press) 75: 59–68. doi:10.1093/mollus/eyn041. ISSN 0260-1230. http://mollus.oxfordjournals.org/cgi/content/abstract/75/1/59 (Abstract).
- ↑ 55.0 55.1 55.2 Iversen, E. S.; Jory, D. E.; Bannerot, S. P. (1986). "Predation on queen conchs, Strombus gigas, in the Bahamas". Bulletin of Marine Science 39(1): 61–75.
- ↑ Jory, D. E. (2006). (Abstract) "An incident of predation on queen conch, Strombus gigas L. (Mollusca, Strombidae), by Atlantic permit, Trachinotus falcatus L. (Pisces, Carangidae)". Journal of Fish Biology (The Fisheries Society of the British Isles) 28 (2): 129–131. doi:10.1111/j.1095-8649.1986.tb05149.x. http://www3.interscience.wiley.com/journal/119489331/abstract (Abstract).
- ↑ 57.0 57.1 57.2 "Virgin Islands Vacation Guide & Community". http://www.vinow.com/articles/101508/conch.php. Retrieved 13 April 2010.
- ↑ 58.0 58.1 58.2 CITES (2003). Review of Significant Trade in specimens of Appendix-II species. (Resolution Conf. 12.8 and Decision 12.75). Nineteenth meeting of the Animals Committee, Geneva (Switzerland), 18–21.
- ↑ NOAA (2009). National Marine Fisheries Service Fisheries Statistics and Economics Division. [1]. Retrieved 4 July 2009.
- ↑ Squires, K. (1941). "Pre-Columbian Man in Southern Florida". Tequesta (Florida International University) (1): 39–46. http://digitalcollections.fiu.edu/tequesta/files/1941/41_1_05.pdf.
- ↑ Turquoise mosaics from Mexico, Colin McEwan, p.32, 2003, British Museum, accessed 29 August 2010
- ↑ 62.0 62.1 Abbott, R. T.; Morris, P. A. (1995). A Field Guide to Shells. New York: Houghton Mifflin Company. pp. 184–185. ISBN 0-395-69779-4.
- ↑ "All About Gemstones". Organic Gems: Conch Pearls. http://www.khulsey.com/jewelry/organic_gems_conch-pearl.html. Retrieved 26 August 2010.
- ↑ Theile, S. (2001). "Queen conch fisheries and their management in the Caribbean". Traffic Europe (CITES): 1–77. http://www.google.com.br/url?sa=t&source=web&ct=res&cd=3&ved=0CB8QFjAC&url=http%3A%2F%2Fwww.traffic.org%2Fspecies-reports%2Ftraffic_species_invertebrates5.pdf&rct=j&q=In+places+where+adults+have+become+rare%2C+juveniles+are+often+harvested+by+conch+fisherman+before+they+have+reproduced&ei=elPES57QOsP48AaRqr3SDw&usg=AFQjCNGC3vifTkx8qEwc5c-GNGIEomHrFg.
- ↑ Oxenford, H. A.; et al (2007). Fishing and marketing of queen conch (Strombus gigas) in Barbados. CERMES Technical Report. 16. University of the West Indies, Barbados: Centre for Resource Management and Environmental Studies. http://www.cavehill.uwi.edu/cermes/Technical_Reports/Oxenford_et_al_2007_Barbados_conch_CRT.pdf.
- ↑ NOAA Fisheries Office of International Affairs website: CITES. Retrieved 4 July 2009.
- ↑ "Standing Committee Recommendations". CITES Official Documents No 2003/057. 2003. http://www.cites.org/eng/notif/2003/057.shtml. Retrieved 16 April 2010.
External links
- ARKive – images and movies of the queen conch (Strombus gigas)
- Animal Diversity Web: Strombus gigas
- Strombus gigas at National Center for Biotechnology Information (NCBI) website.
- Microdocs: Life cycle of the conch
|
|||||